The diseases are not that scary when you actually understand them...Let's compare the risks of each disease for which we vaccinate against the vaccines, one by one.
We are chipping away and examining the vaccines in the strongly recommended schedule. Let's get to it...
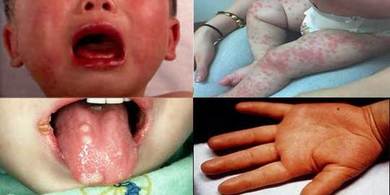
The above photos are of Kawasaki's Disease, a common risk with many vaccines.
H. Influenza Type B (Hib)
Vaccine Timing: 2 months, 4 months, 6 months and 12 months
Risks associated with Hib:
Hib is a type of flu, H. Influenza Type B, which caused bacterial meningitis. It was a nasty disease when it was here (prior to 1990), with about 12,000 cases a year in the US. Of those 12,000 cases, the death rate was approximately 5% and among survivors, approximately 25-35% suffered from neurological complications (often hearing loss). At this particular time, this bacteria is pretty much gone, making the risk of contracting it very low.
Hib Vaccine Ingredients:
Hib (ActHIB)
ammonium sulfate, formalin, sucrose, Modified Mueller and Miller medium
OR
Hib (Hiberix)
formaldehyde, lactose, semi-synthetic medium
OR
Hib (PedvaxHIB)
aluminum hydroxphosphate sulfate, ethanol, enzymes, phenol, detergent, complex fermentation medium
Risks Associated With Hib Vaccine:
Since the bacteria is not here, the molecular mimicry that we discussed in the last post is the primary concern. With an abundance of T-Cells in the body and no bacteria or virus to attack, the T-Cells will start to attack the body instead, causing auto-immune diseases. A study of 200,000 children found that kids with 4 doses of Hib had a 26% higher rate of Type 1 Diabetes which developed 3-4 years post-vaccination. Source. There is now great concern of a penicillin-resistant pneumococci and increased frequency of pneumococcal diseases because of mass Hib vaccination.
Pneumococcal Disease:
Vaccine (PCV 13) Timing: 2, 4, 6, and 12 months
General Information:
PCV13 contains 13 strain of bacteria, so in a sense it is 13 vaccines in one shot.
Risks Associated with Pneumococcal Disease:
VERY low for healthy babies and children. High risk groups include those with predisposing conditions such as immunoglobulin deficiency, Hodgkin's disease, immunodeficiency disorders including HOV, nephrotic syndrome, some viral upper respiratory tract infections, splenic dysfunction, no spleen, and organ transplants. (3)
PCV13 Ingredients:
Here are the carcinogenic and neurotoxic ingredients (5):
casamino acids, yeast, ammonium sulfate, polysorbate 80, succinate buffer, aluminum phosphate, soy peptone broth
Risks Associated with PCV13:
Besides the common fever, change of appetite and sleep, irritability, and muscle and joint pain, the following took place during clinical trials (copied and pasted directly from the package insert):
Serious adverse events reported following vaccination in infants and toddlers occurred in 8.2% among Prevnar 13 recipients and 7.2% among Prevnar recipients.(4)
According to the manufacturer's package insert:
The following adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to Prevnar 13 vaccine.
Administration Site Conditions: Vaccination-site dermatitis, vaccination-site pruritus, vaccination-site urticaria
Blood and Lymphatic System Disorders: Lymphadenopathy localized to the region of the injection site
Cardiac Disorders: Cyanosis
Immune System Disorders: Anaphylactic/anaphylactoid reaction including shock
Nervous System Disorders: Hypotonia
Skin and Subcutaneous Tissue Disorders: Angioneurotic edema, erythema multiform
Respiratory: Apnea
Vascular Disorders: Pallor
There were 3 (0.063%) deaths among Prevnar 13 recipients, and 1 (0.036%) death in Prevnar.
All 4 events (hypotonic-hypononresponsive episode) occurred in a single clinical trial in Brazil in which subjects received whole cell pertussis vaccine at the same time as Prevnar 13 or Prevnar.
Reactions occurring in greater than 1% of infants and toddlers: diarrhea, vomiting, and rash. Reactions occurring in less than 1% of infants and toddlers: crying, hypersensitivity reaction (including face edema, dyspnea, and bronchospasm), seizures (including febrile seizures), and urticaria or urticaria-like rash.
When we keep hearing from our pediatricians, the media, the CDC, and the FDA that vaccine are so harmless that only 1:1,000,000 have reactions it might be easy to feel caught off-guard and silly if you are not prepared. Is that really true...1 in a million??? No.
Almost 1% of children vaccinated (.8%) with an aluminum-containing vaccine in a study had intense, long-lasting (75% still had symptoms after 4 years) itchy nodules. (5)
The amount of aluminum in a vaccine is not supposed to exceed 1.25mg. On any given scheduled vaccination day a child commonly would get at least 4 vaccines containing aluminum which could bring the dose of aluminum to as much as 1.475mg and this schedule is repeated several times throughout the childhood vaccine schedule. The Hepatitis B vaccine alone has .5mg of aluminum hydroxide. (5)
Aluminum is eliminated from the body primarily via kidneys, which are not at full-capacity until 1-2 years of age. When aluminum enters the bloodstream it exhibits neurotoxic behavior and where it can enter the brain and inhibit the blood brain barrier (BBB) functions which result in significant neuron damage to the CNS (Central Nervous System). This damage removes the protection of the brain from other toxins in the blood. (5)
As a perspective on a risks vs. benefits piece, I just need to state the obvious here. We are knowingly injecting our infants several times during the first 2 years of life before their kidneys are fully-functional (and are capable of removing it) with a substance that is KNOWN to cause brain damage, as described above, not knowing the long-term consequences of this action because double blind studies have never been done on vaccines. To reiterate, there have never been double blind studies on vaccines to observe anything, including kidney damage, long-term brain function, learning abilities, IQ impact, or disease rates. We do this in order to "protect them" against illnesses for which they have little or no risk. And we do this knowing that there are some risks, many actually, that are not fully-disclosed to parents by our pediatricians, who repeatedly tell us they are perfectly safe. They can say that confidently, knowing that they can never be held liable if your child has a reaction to the vaccine.
Rotavirus:
Vaccine Timing: 2, 4 , and 6 months
Risks Associated with Rotavirus:
Rotavirus is a virus that causes diarrhea, vomiting, and low-grade fever. It is spread from feces to mouth in young children. Prognosis? Excellent. There is no medication for aside from hydration and electrolytes. In breast-fed children the risk is lower because the babies usually receive antibodies from mother's milk. Where medical care is readily accessible, including hydration therapy if needed, full recovery can be expected. In developing countries where hydration therapy (or even clean water) is not available, children can die from dehydration.
Rotavirus Ingredients:
Rotavirus (RotaTeq)
sucrose, sodium citrate, sodium phosphate monobasic monohydrate, sodium hydroxide, polysorbate 80, cell culture media, fetal bovine serum, vero cells [DNA from porcine circoviruses (PCV) 1 and 2 has been detected in RotaTeq. PCV-1 and PCV-2 are not known to cause disease in humans.]
OR
Rotavirus (Rotarix)
amino acids, dextran, sorbitol, sucrose, calcium carbonate, xanthan, Dulbecco’s Modified Eagle Medium (potassium chloride, magnesium sulfate, ferric (III) nitrate, sodium phosphate, sodium pyruvate, D- glucose, concentrated vitamin solution, L-cystine, L-tyrosine, amino acids solution, L-glutamine, calcium chloride, sodium hydrogenocarbonate, and phenol red) [Porcine circovirus type 1 (PCV-1) is present in Rotarix. PCV-1 is not known to cause disease in humans.]
Risks Associated with the Rotavirus Vaccine (6):
The vaccine contains live virus, so the baby being vaccinated can contract the disease from the vaccine and also spread the disease. Common side-effects include...are you ready??? diarrhea, vomiting, fever, and stomach pain. Other risks associated with this vaccine include:
Gastrointestinal Disorders: Intussusception (including death), recurrent intussusception (including death), hematochezia, gastroenteritis with vaccine viral shedding in infants with Severe Combined Immunodeficiency Disease (SCID).
Blood and Lymphatic System Disorders: Idiopathic thrombocytopenic purpura. Vascular Disorders: Kawasaki disease.
Special Note and Call to Action...
Rotavirus Vaccine was developed and pioneered by Dr. Paul Offit. Offit is a name you need to remember if you don't already know it. He is hailed as a vaccine hero among pro-vax media, pharma, physicians and hospitals. He is a vaccine bully who advocates removal of faith-based exemption of vaccines, calling it neglect. He is staunchly against dietary supplements and alternative medicine and it appears he would like to see our free-choice revoked regarding vaccines and also eliminate our choices regarding all holistic healthcare. He made the claim that an infant could safely withstand 100,000 vaccines at once. This was printed in the CHOP Parents Pack, link here. I would like to challenge Paul Offit, and any and every doctor that supports his views to lead by example. Help reduce our concerns by receiving 100,000 vaccines at once to show us first hand that they are not harmful.
Please, Mr. Offit (and any/all of his supporters) go on national television and receive 100,000 vaccines. I later heard you changed that to 10,000 vaccines. Again, if you have decided that 100,000 is too many, but 10,000 is safe for an infant...prove it. Go on 20/20, Dr. Oz, FOX, Dateline, the News or any other program and get 10,000 vaccines at once and then let the public follow your health journey so that we can see just how safe it is. If you survive, show us with regular check-ups that you will not develop diseases that give rise for concern among the vaccine-resistant population such as cancer, diabetes, Kawasaki's, arthritis, brain damage, or any number of other autoimmune disorders or diseases. We want to see that your kidneys function perfectly and there are no implications of Alzheimer's following or cancer in your near future. If you really want to impress us, you could have full blood-work, scans, and the like before and then periodically in the years following to prove no harm has come your way from vaccine doses you claim to be safe for a developing infant. Be willing to back up your own statement and put your health on the line and do this yourself. Maybe, just maybe, if we could get you and even 20, out of the tens of thousands of pro-vaccine physicians, to televise such an event by all getting 100,000 (or 10,000 if you are afraid of 100,000) vaccines to prove just how safe they are, and we could follow-up by checking in every month for 5 years (many things like Type 1 Diabetes shows up after 3-4 years), if we could see that you are all in perfect health, disease-free, I might start to think they are not as harmful as I thought and reconsider my position. It would be a great opportunity for you to prove to the masses you consider so foolish and negligent that you are right and vaccines are perfectly safe. Unfortunately, the odds of any doctor, including the one who made the claim, stepping up to this challenge are highly unlikely.
Stay tuned for Part 3, coming soon...
Courtney Charles is a frequent contributor for TruthKings. Connect with her at Alkaline Mom or on her website alabasterliving.com.
References:
1. Ref: Journal of Autoimmunity 35:247-253, 2002.
2. June 1992 Newsletter from Journal of Pediatric Infectious Disease
3. 1994 Red Book Report of the committee on Infectious Diseases
4. CDC, ingredient list
5. Dr. Sherri Tenpenny citing: Simmer, K. Aluminum in Infancy. In:Zatta PF, Alfrey AC. (Eds) Aluminum Toxicity in Infants' Health and Disease. 1997, World Scientific Publishing.; Source: Berfors E, Vaccine. 2003 Dec 8;22(1):64-9
6. Rotavirus package insert

 RSS Feed
RSS Feed